At the moment, power stations burn methane from natural gas as fuel.
CH4(g) + 2O2(g) ——-> CO2(g) + 2H2O(l)
In Scotland, they are developing decarbonised fuels to reduce carbon dioxide emission.
CH4(g) + 2H2O(g) ——-> CO2(g) + 4H2(g)
The hydrogen produced is used in generating electricity and the carbon dioxide stored away. This way, more energy is produced without having to produce as much CO2.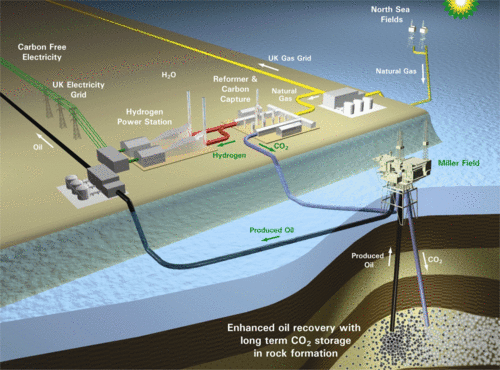

Storage as carbonates
CO2 can also be stored as carbonates by reacting them with metal oxides. The carbonates would be stable and happens naturally.
CaO(s) + CO2(g) ———> CaCO3(s)
However, it takes a lot of energy to speed up this slow natural process so more research has to be carried out in order to make this technique more efficient.